- Have Revised
Device Regulation¶
Before any medical device can be applied on patients it must be regulated to confirm its safety
The relevant regulatory bodies:
- UK: MHRA
- US: FDA
Getting approval for a biomedical device is quite a laborious procedure.
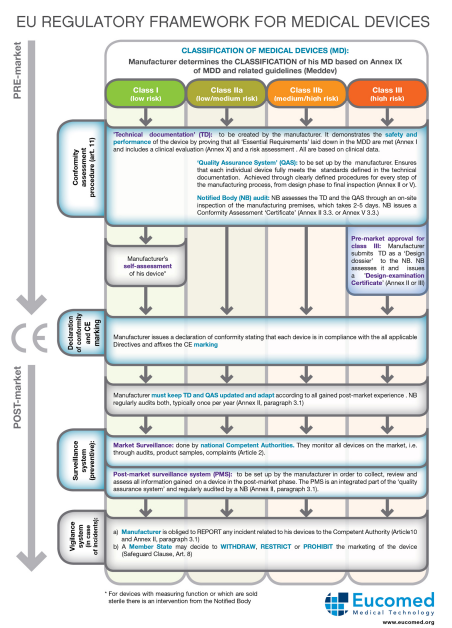
This procedure is very thorough and requires a lot of technical documentation, explaining how it works, it needs significant testing to prove that it’s safe to the regulators. However this doesn’t end once you’ve got the approval, you still need to have a quality management system in place as they will be constantly monitoring the process of making the devices, it will be routinely investigated.
Medical Device Definition¶
A ‘Medical Device’ means any instrument apparatus, appliance, material or other article, whether used alone or in combination, including software necessary for its proper application intended by the manufacturer to be used for human beings for the purpose of:
- diagnosis, prevention, monitoring, treatment or alleviation of disease
- Diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap
- investigation, replacement or modification of the anatomy or of a physiological process
- Control of conception
and which does not achieve its principle intended action in or on the human body by pharamacological, immunological or metabolic means, but which may be assisted in its function by such means;
Active Medical Device¶
’Active medical device’ means any medical device relying for its functioning on a source of electrical energy or any source of power other than that directly generated by the human body or gravity;
’Active implantable medical device’ means any active medical device which is intended to be totally or partially introduced, surgically or medically, into the human body or by medical intervention into a natural orifice, and which is intended to remain after this procedure
In Vitro Diagnostic¶
“in vitro diagnostic medical device” means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, equipment, or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information:
- Concerning a physiological or pathological state, or
- Concerning a congenital abnormality, or
- Determine the safety and compatibility with potential recipients, or
- To monitor therapeutic measures..."
Classes - MHRA¶
We classify the aforementioned device types in 4 classes.
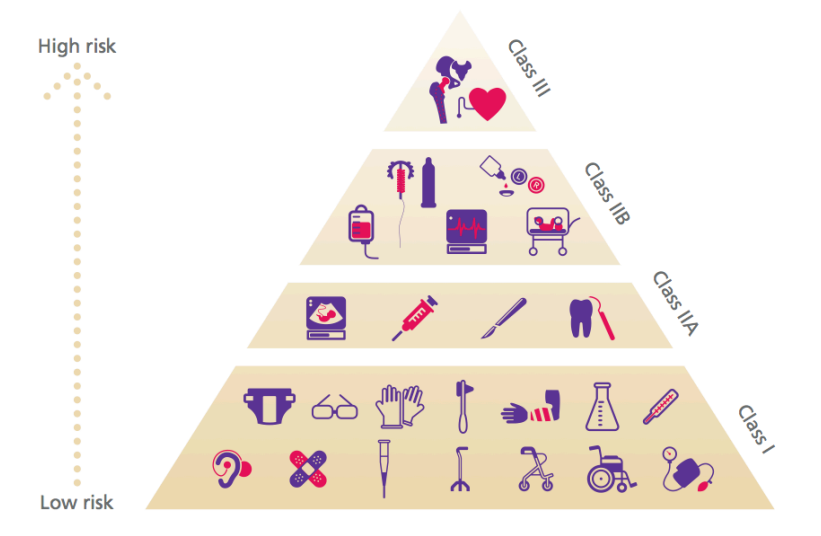
- Class I
- generally regarded as low risk
- Example: Hearing aid
- Class II a
- generally regarded as medium risk
- Example: Continuous glucose monitors
- Class II b
- generally regarded as medium risk (can potentially be harmful)
- Example: Artificial Pancreas, ECG
- Class III
- generally regarded as high risk (implantable)
- Example: Pacemaker, hip replacement
The Rules (UK)¶
• Competent Authority (UK Bulleetin No. 10): THE RULES • Rules 1-4 - non-invasive devices • Rules 5-8 - invasive devices • Rules 9-12 - additional Rules applicable to active devices • Rules 13-18 - miscellaneous Rules for products which merit a higher classification than they might otherwise be assigned